Is your medical device on the market, but you’re not sure what to do next? It’s easy to feel overwhelmed by the sheer complexity of conducting a post-market clinical follow-up (PMCF). But by breaking the process down into manageable steps, we offer a straightforward guide to help manufacturers adhere to regulatory requirements. Follow the steps in this guide to ensure the ongoing safety and performance of your medical device.
Medical device manufacturers must create, define, and conduct post-market clinical follow-up activities according to the EU MDR. This involves the creation of a PMCF plan and a follow-up report to identify risks of a CE-marked device and to determine its long-term clinical performance.
What is a post-market clinical follow-up?
Post-market clinical follow-up (PMCF) is a proactive process that medical device manufacturers use to gather data on clinical experience with their devices after they have been released on the market. This process is critical to ensuring the ongoing safety and performance of a device throughout its entire lifecycle.
The goal of a PMCF is to systematically collect, record, and analyze clinical data relating to a device after it is released on the market. This process aims to:
- Continuously assess the device’s long-term safety and performance
- Validate and update the clinical evaluation and detect rare adverse events
- Identify any need for preventive, corrective, or field safety corrective action
- Evaluate the impact of any changes in the device, its manufacturing process, or use
When must manufacturers carry out a PMCF?
According to the EU Medical Device Regulation (MDR), manufacturers must begin a PMCF in the following circumstances:
- When there is a need to confirm the safety or performance of the device over a longer period than was possible during the pre-market phase
- When the device is intended to treat, diagnose, or monitor a life-threatening or chronically debilitating condition
- If the device is expected to have a significant public health impact
- If the device is intended to be implanted for at least 30 days
- If there is a high product risk classification
- If the device is of an innovative nature
- When the device is not fully represented, or not represented at all, in the clinical evaluation for the device category or the equivalent device range
However, a manufacturer might not need to conduct a PMCF if the device has been on the market for a long-enough period where there is sufficient data available from its post-market surveillance, if there is sufficient clinical evidence for similar devices, or if the device’s risk level is low and unlikely to result in serious harm.
The decision to not conduct a PMCF must be justified and documented in the clinical evaluation report. However, the need for a PMCF may change over time as new information about the device or its use becomes available. Therefore, the decision to carry out a PMCF must be periodically reassessed.
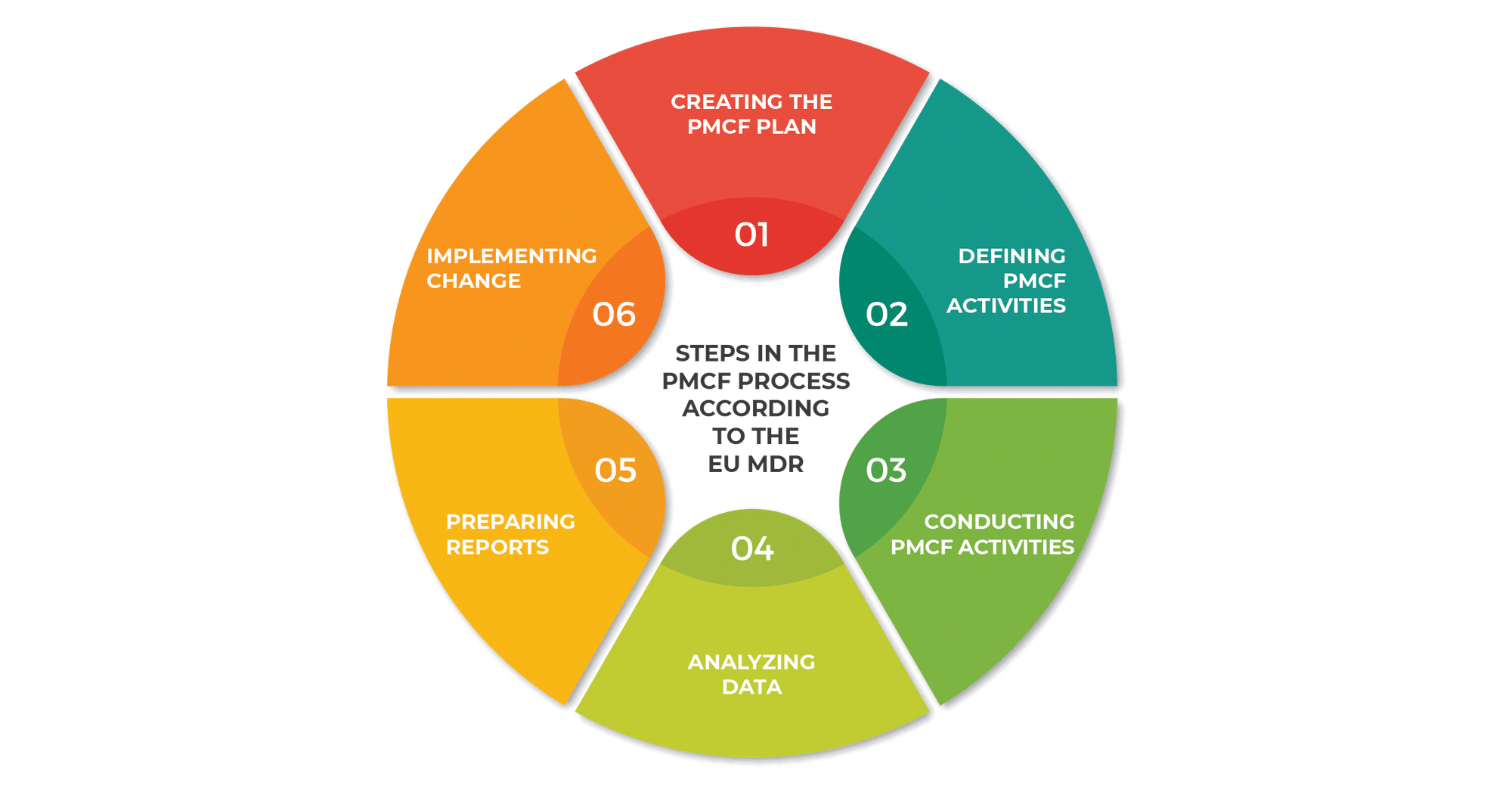
Steps in the PMCF process according to the EU MDR
The EU MDR outlines a series of steps for carrying out the post-market clinical follow-up process, for manufacturers to continually monitor device safety and performance.
1) Creating the PMCF plan
The first step involves designing a comprehensive PMCF plan (according to MDCG 2020-7, a guide published by the Medical Device Coordination Group). It should outline:
- The methods and procedures for collecting and analyzing clinical data
- The timeline for the PMCF activities
- The roles and responsibilities of those involved
The purpose of the plan must also be made clear. For instance, a manufacturer of a pacemaker wants to see if there is any new information that can jeopardize the patient’s safety and understand the performance constraints of the device.
Discover how to design a comprehensive PMCF plan with this free downloadable template: Post-Market Clinical Follow-Up (PMCF) Plan Template.
2) Defining PMCF activities
The next step is to define which PMCF activities will be used to collect necessary clinical data. These activities can be clinical investigations, case studies, surveys, scientific literature research, and registries.
One example of an activity is a planned real-world evidence (RWE) analysis, which would include a summary of the plan, including the design, sample size, and population analysis. Additionally, it should define how data from reliable sources will be gathered and utilized.
3) Conducting PMCF activities
When the activities have been clearly defined, further supplementary information must be provided, including:
- A proper description
- An aim (for example, confirming the safety and performance of the device, identifying previously unknown side effects, etc.)
- Information pertaining to different PMCF procedures (for example, screening of scientific literature and other sources of clinical data, post-market studies, and collecting data in registries)
- Rationale for the appropriateness of the chosen methods and procedures.
Once the above elements are addressed, the PMCF activities can be conducted in accordance with regulatory requirements.
4) Analyzing data
Once the activities have been carried out, data must then be collected and thoroughly analyzed to identify any emerging risks, and to ensure the continued acceptability of identified risks.
Research methods used in PMCF studies can include clinical investigation or an analysis of patient registries (like the current MAUDE [USA patient registry] or MHRA [UK patient registry] and, in the future, EUDAMED). Some examples of research methods are:
- Pre-market clinical trials
- Patient registries
- Surveys and interviews
- Complaint and service records
The data must then be compiled and analyzed in a way that enables the researchers to draw conclusions from it. For example, information about the fracture and the short battery life of a pacemaker can be gathered from:
- Complaint data, to learn about device malfunctions and user concerns
- Patient registries, to see if competitors have reported similar problems
- Interviews with patients, to determine whether they follow all the maintenance instructions
By using different statistical methods and trend analyses, a manufacturer can develop recommendations to mitigate the associated risks. Such methods may involve calculating measures of central tendency (like means and medians) and measures of dispersion (like range and standard deviation) to understand the general trends and variability in the data.
5) Preparing reports
According to MDCG 2020-8 (another guide published by the Medical Device Coordination Group), the PMCF evaluation report must provide a clear and detailed summary of the data analysis and discuss any implications for the safety and performance of the device. The results of the PMCF must also be used to update other relevant documentation, such as the device’s clinical evaluation report and risk management file.
Finally, the PMCF plan must be reviewed and updated regularly to ensure it remains relevant in light of any new information or changes to the device or its use.
6) Implementing change
Based on the results from the PMCF, manufacturers can make several decisions to enhance their products and adhere to regulatory requirements.
For example, if the PMCF for a pacemaker reveals that patients require longer battery life, manufacturers may choose to revise the device design, update the usage instructions, or improve the manufacturing processes.
If new risks are identified during the PMCF, like an increase in fractures or damage to the pacemaker, manufacturers may need to implement additional control measures or revise their risk-benefit analysis. This might result in updating the device labeling to provide clearer instructions or additional warnings.
The clinical evaluation report must reflect any new concerns obtained during the PMCF about the device’s safety or performance. Once the process is complete, the manufacturer will need to complete a periodic safety update report.
Post-market clinical follow-up plan structure
The PMCF plan must be a live document that is updated as new information becomes available. The structure of the follow-up plan proposed by the Medical Device Coordination Group in MDCG-2020-7 is:
- Section A. Manufacturer contact details
- Section B. Medical device description and specification
- Section C. Activities related to PMCF: general and specific methods and procedures
- Section D. Reference to the relevant parts of the technical documentation
- Section E. Evaluation of clinical data relating to equivalent or similar devices
- Section F. Reference to any applicable common specification(s), harmonized standard(s) or applicable guidance document(s)
- Section G. Estimated date of the PMCF evaluation report
To help you implement your PMCF plan, download a free template here: Post-Market Clinical Follow-up (PMCF) Plan Template.
Post-market clinical follow-up report structure
A comprehensive PMCF report is a vital part of the post-market clinical follow-up process. The structure of the follow-up report proposed by the Medical Device Coordination Group in MDCG-2020-8 is:
- Section A. Manufacturer contact details
- Section B. Medical device description and specification
- Section C. Activities undertaken related to PMCF: results
- Section D. Evaluation of clinical data relating to equivalent or similar devices
- Section E. Impact of the results on the technical documentation
- Section F. Reference to any common specification(s), harmonized standard(s) or guidance document(s) applied
- Section G. Conclusion
The objective of these templates is to aid manufacturers in fulfilling the obligations of the MDR. By presenting post-market clinical data in an organized and comprehensive manner, manufacturers can streamline the review process for notified bodies and competent authorities.
The importance of PMCF
With the results obtained from the PMCF, manufacturers gain valuable insights into the real-world performance of their devices. This includes understanding usage patterns, identifying potential improvements, and confirming the safety and effectiveness of the device in a broader patient population.
These insights can guide future product enhancements and aid in the development of new devices. Furthermore, compliance with PMCF requirements can help manufacturers meet regulatory obligations, maintain market access, and build trust with patients, healthcare providers, and regulators.
To prepare your PMCF documents and policies in compliance with the EU MDR in the fastest and most efficient way, check out this EU MDR & ISO 13485 Integrated Documentation Toolkit.


 Kristina Zvonar Brkic
Kristina Zvonar Brkic