There are many ways to structure the documentation required for an aerospace Quality Management System (QMS) that will meet the requirements of AS9100 Rev D, but many people think that this means the documentation must be bureaucratic and wordy. While it is true that AS9100 Rev D requires some documentation (see List of mandatory documents in AS9100 Rev D), this does not mean that the documentation system needs to be extensive. The QMS documentation is there to provide the organizational framework for the QMS, consistency in processes, and evidence that the goals were achieved. How you structure the QMS documentation is, ultimately, up to you.
Hierarchy of QMS documents
One common method of structuring the QMS documentation is to have a hierarchy of documents, with the lower-level documents being more specific and referring to the upper-level documents that provide guidance on the QMS. The hierarchy is often shown in the following diagram:
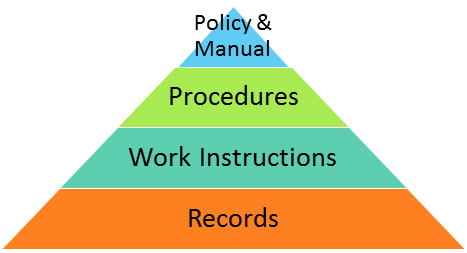
While there are different types of documented information required by AS9100 Rev D, not all identified information needs to be documented as separate documents. The standard requirements are flexible enough that you can organize your QMS documentation however you wish, but there are some guidelines available.
Structuring QMS documentation
There is an international standard, ISO 10013:2001, which provides guidelines for Quality Management System documentation. While this standard has not yet been updated in light of the newly released QMS requirements, it is still a good guide on structuring documentation. These recommendations take the above guidelines into account:
1) Quality policy & manual – The quality policy is the declaration for how your company intends to provide products and services that meet requirements in such a way that you will enhance customer satisfaction and continually improve—a guidance to all further initiatives in the QMS.
The quality manual, which has been the backbone of the QMS until the recent revision changes, is no longer a requirement in AS9100 Rev D. However, it is mentioned as a useful document for companies to incorporate, and is a good place to collect the following required information: general description of relevant interested parties, QMS scope including boundaries and applicability, description of QMS processes and application, sequence and interaction of QMS processes, and assignment of responsibilities and authorities for QMS processes. If used, the manual should fit your organizational needs and complexity.
Find out more in this article: The future of the quality manual in AS9100.
2) Quality procedures – Many formats and structures for quality procedures exist, such as narrative texts or flowcharts, but there are a few key elements that should be included:
- Title – To identify the procedure for the reader.
- Document control – What changes have happened to this procedure, and when? Who approved the changes? What is the current revision of this document?
- Scope and purpose – What is covered in the procedure, and why does this procedure exist?
- Responsibilities and authorities – Who is part of the procedure, and what are they allowed to do?
- Activities – What happens in the procedure? This is the main section of the procedure and describes what needs to be done. What should be done? Who should do it? How should it be done? When and where should it be done? The inputs and outputs of the process should also be captured in this section, as well as required resources.
- Records – What evidence needs to be kept to demonstrate the procedure has been done correctly?
- Appendices – This may be included if necessary, such as a table that could be referenced to perform the activities.
3) Work instructions – While these are sometimes included in a procedure, it is often necessary to have separate step-by-step instructions on how to perform a particular task. The structure of work instructions can be the same as the procedures; however, the activities section will focus on the details necessary to ensure activities are realized. This can include sequential steps, tools required, and methods to be used. The level of detail in your work instructions is directly related to the competencies required to perform the process.
4) Records – Records can take many forms and, as identified in the procedure, are the evidence that is needed to demonstrate that the procedure was done correctly. Records can start as forms that are filled in and approved, reports that are created from test equipment, or minutes that are kept from meetings. The most important thing to remember about records is that they are there to show conformance, and then need to be safeguarded so that they can be retrieved and reviewed if needed.
For an effective QMS, ensure you have a functioning documentation system
It is important to remember that the documentation system is there to serve you and the QMS, not the other way around. You want to create a documentation system that will satisfy your needs, make your operations easier to perform and review, and satisfy the needs of your customers. If you find that your documentation system is forcing you to change processes that are meeting all other requirements, then you might want to review the documentation system. A poorly implemented documentation system can cause problems that you just don’t need.
To find out more about AS9100 Rev D and how it works, see this link: What is AS9100?


 Mark Hammar
Mark Hammar 


